White House Misses Golden Opportunity to Push For Meat Raised Without Abusing Antibiotics

The White House scored some points for creativity in trying to address the national security threat of growing antibiotic resistance. Unfortunately, it missed the mark in some key areas.
Let's break down what they did:
Federal Procurement of "Responsibly" Raised Meat
Of the three things the White House did today, the procurement policy was most interesting and held the greatest potential. A well-framed procurement policy can really drive market innovation by creating the incentive for companies to improve their practices. But a procurement policy that supports continued misuse of antibiotics doesn't accomplish much at all--and this policy supports producers that use antibiotics routinely in meat and poultry production.
The White House is requiring all the federal departments and agencies to "create a preference for meat and poultry produced according to responsible antibiotic use..." by using the federal government's considerable purchasing power to offer these options. Specifically, the government will begin to make meat and poultry raised "according to responsible antibiotic-use policies" available over the next few years to both federal cafeterias and facilities.
But the devil is in the details. In this case, the details about what constitute "responsible" antibiotic-use policies undermine the chance that this plan will accomplish much.
The White House defined the "responsible" use of antibiotics the same way it defined "judicious" use under the FDA guidance. Under this definition, "responsible" or "judicious" use would not allow routine use of antibiotics for growth promotion, but would allow other routine uses of antibiotics such as for disease prevention.
Unfortunately, since disease prevention and growth promotion uses are very similar (and oftentimes identical), the actual on the farm practices regarding antibiotic use will not likely change much and antibiotic use on the farm will not significantly decline. Routine, habitual, use of antibiotics will continue.
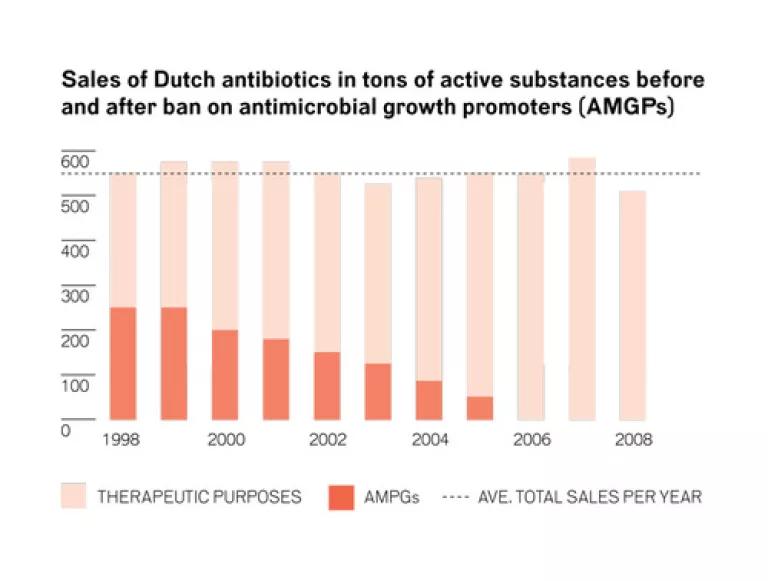
The Netherlands provides a great example of this. There, they first focused exclusively on reducing growth promotion uses (as the FDA does here), but it failed to put a dent in overall antibiotic overuse in food animals. Once authorities instituted a set of additional policy reforms, including specifically targeted other routine antibiotic uses - that is, for disease prevention - they began to reduce overall livestock usage. Today, they have reduced animal use of antibiotics by 50%.
Source: Maryn McKenna
In fact, we don't even have to go as far as the Netherlands to see that more can be done. The federal policy lags behind that of major poultry companies like Tyson Foods and Perdue, which have committed to or are eliminating all uses of medically important antibiotics except for cases to treat sick animals. In other words, these giant corporations are not using medically important antibiotics day after day.
If the new federal procurement policy in the U.S. is intended to spur market innovation, then it needs to create preferences for meat and poultry that exceed - not just meet - the low and ineffective federal requirements. As written, the procurement policy essentially has a preference for purchasing meat and poultry that meet FDA's weak guidance. Since FDA's weak requirements are expected to be mandatory by December 2016 for all livestock producers, the federal purchasing policy merely gives a preference for meat products that comply with the law. It doesn't change the status quo at all.
Finalization of Veterinary Rules on Feed Containing Antibiotics
The White House also announced the long awaited release of the FDA's final Veterinary Feed Directive, a rule governing how veterinarians authorize, or prescribe, ongoing uses of antibiotics in animal feed.
There were some improvements compared to the proposed rule. For example, it's great that FDA decided to preserve an existing requirement that veterinarians have knowledge of the animals in question before they place an order for an antibiotic or other drug. FDA also removed a loophole that allowed drug manufacturers to market the growth promotion benefits of their animal feed drugs, even though the companies were no longer allowed to sell those drugs with growth promotion on the drug label.
However, FDA has missed another opportunity to collect antibiotic use data with the release of this rule. These use data are important to track whether any of these programs are working to reduce antibiotic use significantly.
Industry Forum on the Misuse of Antibiotics
Finally, the White House convened 150 stakeholders involved in the use and misuse of antibiotics on both the human and the animal side. Presumably, this event was intended to highlight the business case for protecting antibiotics and discuss the consumer pressure to shift away from the overuse of antibiotics.
Citing Panera, Tyson, and McDonald's (among others), the Administration touted private sector commitments that will help improve "responsible" antibiotics use. Notably, these companies' commitments surpass the federal government's own (low) standard of responsible antibiotic use. Companies like Tyson and Foster Farms establish the basic standard that all companies should be meeting: that is, only using "medically important" antibiotics to treat animals that are sick or to control disease and striving to eliminate the use of medically important antibiotics altogether. Companies like Panera are going even further in their policies and commitments to purchase meats raised without any antibiotics.
The White House acknowledged that more work needs to be done to strengthen antibiotic stewardship programs. Hopefully, this forum will give the Administration and companies the push needed to make real, meaningful changes to how we use antibiotics in raising livestock, so that we can preserve these important life-saving medicines.
