Hydrogen Safety: Let's Clear the Air
When handled responsibly, green hydrogen is less dangerous than other flammable fuels that we rely on today. Moving forward, industry and government institutions must build on existing robust safety protocols and continue to make safety a key priority for investment and refinement to ensure that hydrogen becomes part of a clean and thriving economy.

Credit: iStock/audioundwerbung
This blog is the first in a series exploring the safety considerations of energy technologies critical for a carbon-neutral economy.
Many experts agree “green” hydrogen produced using renewable energy can play a key role in helping us achieve a greenhouse gas-neutral economy by 2050, the level needed to stave off the worst effects of the climate crisis. Green hydrogen’s best use may be in hard-to-electrify sectors, like long-distance transportation, but some people are concerned it also could create new safety risks—even though hydrogen generally has properties that make it safer to handle than conventional fuels.
Hydrogen—mostly used in refineries and fertilizer production today—is currently produced via a dirty process that relies on fossil gas as feedstock and emits a significant amount of carbon pollution. However, the process can be cleaned up to produce a “green” version using renewable energy and water.
Green hydrogen—being produced in only small amounts today—has the potential to replace fossil fuels in emissions-heavy vehicles like trucks, ships, and planes, and in industrial processes like the production of steel and chemicals. It can help us achieve a 100 percent renewable energy electricity sector by allowing us to store power for long periods of time. The gas industry is even pushing hydrogen as a clean substitute for fossil gas for heating spaces and water in buildings, although it would be an expensive solution compared to transitioning our buildings to operating on electricity. (See this blog.)
Safety questions?
Some skeptics point to hydrogen’s safety issues as a potential deal-breaker to its more widespread use. While hydrogen has known safety hazards and that we should continue to prioritize hydrogen safety measures, it also has properties that should make it safer to handle than conventional fuels like gasoline and diesel when handled responsibly.
Hydrogen is the universe’s most abundant and simplest element. On earth, it mainly exists as an essential component of water (H2O). Hydrogen gas (H2) is composed of two hydrogen atoms stuck together, each containing just one proton and one electron. This simple chemical structure is what makes hydrogen gas flammable and relatively easy to ignite. This is also why hydrogen gas is non-toxic, odorless, tasteless, and light.
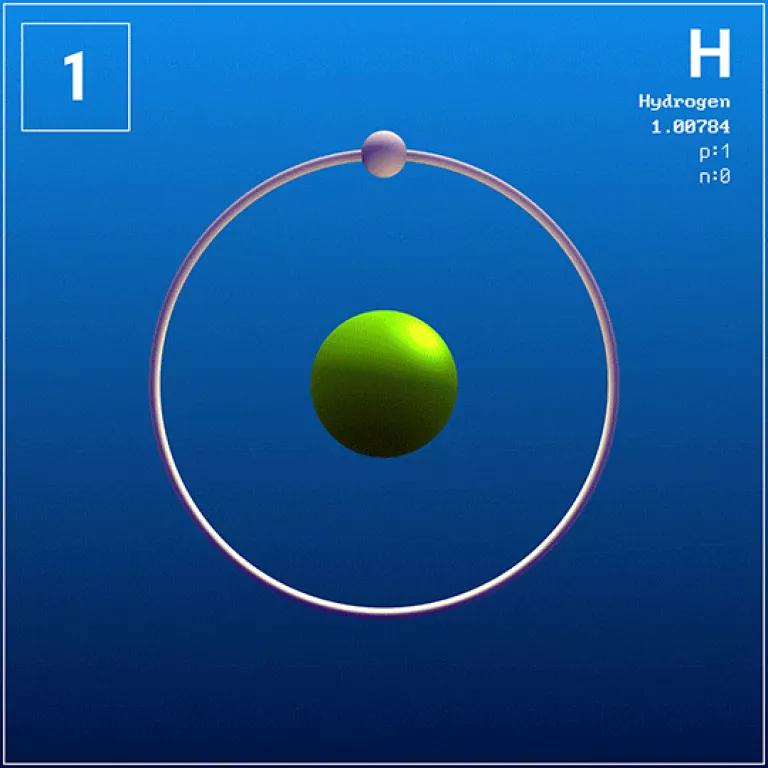
Chemical structure of a hydrogen atom
Wikimedia/SE3-29X
Green hydrogen is safer than conventional fuels
To evaluate hydrogen’s safety, it must be compared to that of other conventional fuels like gasoline, propane, and diesel. While no fuel is 100 percent safe, green hydrogen has been shown to be safer than conventional fuels in a multitude of aspects.
- Hydrogen is not toxic, unlike conventional fuels. On the other hand, many conventional fuels are toxic or contain toxic substances, including powerful carcinogens. Moreover, when it comes to vehicles that run on hydrogen fuel cells, hydrogen produces only water, while vehicle combustion of conventional fuels generates harmful air pollution. A hydrogen leak or spill will not contaminate the environment or threaten the health of humans or wildlife, but fossil fuels can pose significant health and ecological threats when leaked, spilled, or combusted.
- Hydrogen is 14 times lighter than air and 57 times lighter than gasoline vapor. This means that when released, hydrogen will typically rise and disperse rapidly, greatly reducing the risk of ignition at ground level. However, propane and gasoline vapor are heavier than air, making it more likely that they will remain at ground level, increasing the risk of fires harming people and buildings.
- Hydrogen has a lower radiant heat than conventional gasoline, meaning the air around the flame of hydrogen is not as hot as around a gasoline flame. Therefore, the risk of hydrogen secondary fires is lower.
- Hydrogen has a higher oxygen requirement for explosion than fossil fuels. Hydrogen can be explosive with oxygen concentrations between 18 and 59 percent while gasoline can be explosive at oxygen concentrations between 1 and 3 percent. This means that gasoline has greater risk for explosion than hydrogen for any given environment with oxygen.
Hydrogen safety standards have come a long way
Although there has been a lot of recent hype around hydrogen, it is not a new technology. Industry has been using hydrogen in rocket fuel, oil refineries, and fertilizer production for the past 40 years—more than enough time for scientists and engineers to develop and adopt robust safety protocols. Today, the Hydrogen Industry Panel on Codes, International Code Council, and National Fire Protection Association work together to develop stringent standards for hydrogen systems and fuel cells. Years of R&D and experience have made it possible to develop the appropriate engineering controls and guidelines to mitigate the risks of hydrogen’s high flammability and low ignition energy (the energy required to ignite something).
For example, because hydrogen is colorless and odorless, sensors are a requirement for hydrogen fueling stations, equipment, and facilities. Today’s technology enables remote hydrogen sensing to ensure robust detection of any hydrogen leak. Hydrogen storage tanks in fuel cell cars are also subject to rigid testing standards, such as exposure to extreme temperatures and pressures, before they can be deployed. These are just a few examples of the standards and codes that have supported a safe hydrogen industry for the last four decades.
However, and importantly, hydrogen safety continues to be a key priority for R&D. At the federal level, the Department of Energy funds hydrogen safety R&D projects within its Hydrogen and Fuel Cell Technologies Office, contributing to a future where hydrogen can continue to be a safe fuel that can help decarbonize our future energy needs.
Hydrogen safety should continue to be a priority
While green hydrogen offers a solution for the hardest-to-abate sectors, we have little experience using it in those applications. This is why hydrogen safety research is critical if it is to play a bigger role in our economy.
Storing hydrogen in tanks within fuel-cell trucks and hydrogen-powered airplanes—in a cost-effective way without sacrificing safety—presents an engineering challenge. Both the fuel cell trucking and aviation industries will need to achieve similar or better safety targets than those of fossil fuel-burning trucks and aircraft, given that transportation safety of any kind is extremely important. New hydrogen refueling stations and hydrogen pipelines must be engineered in a way that mitigates any risk of dangerous leakage and combustion. Groups like the Center for Hydrogen Safety (CHS), a global non-profit dedicated to promoting hydrogen safety, are tackling these issues by providing resources to those tasked with designing or using various hydrogen systems and facilities, as well as training for incident response. With 45-plus member organizations within CHS (Shell, Hyundai, Argonne National Laboratory, National Grid, to name a few), industry is prioritizing safety.
The verdict
When handled responsibly, green hydrogen is less dangerous than other flammable fuels that we rely on today. Moving forward, industry and government institutions must build on existing robust safety protocols and continue to make safety a key priority for investment and refinement to ensure that hydrogen becomes part of a clean and thriving economy.




