Reporting requirements in new DATA Act a common sense step towards regulating antibiotics in meat industry
Here’s what we know:
Antibiotic resistant infections are on the rise. The Centers for Disease Control warns that dangerous bacteria are increasingly resistant to our arsenal of antibiotics. The World Health Organization (WHO) has warned, “Things as common as strep throat or a child’s scratched knee could once again kill…a post-antibiotic era means, in effect, an end to modern medicine as we know it.”
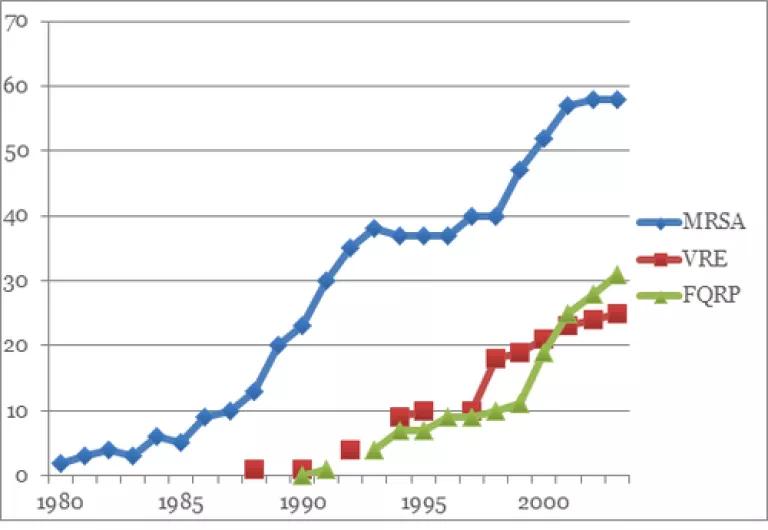
Source: Data collected from hospital intensive care units that participate in the National Nosocomial Infections Surveillance System of the Centers for Disease Control.
At the same time, few new antibiotics are being developed.
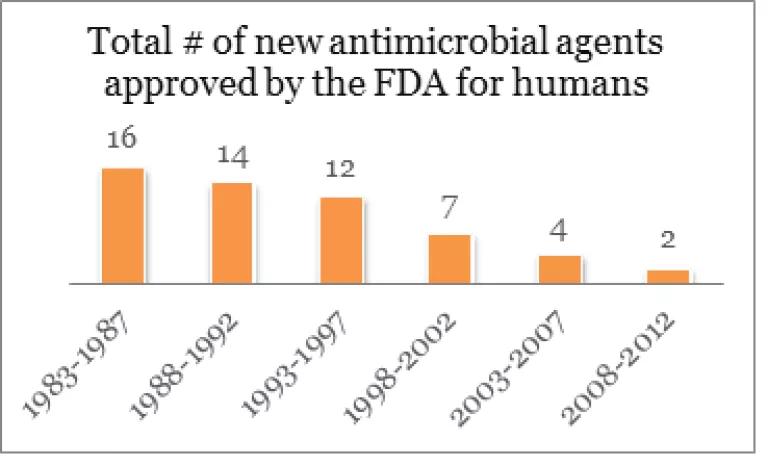
Source: Infectious Diseases Society of America Clin Infect Dis. 2011; 52:S397-S428
According to the Food and Drug Administration (FDA), 70% of all medically important antibiotics sold in the U.S. are used in food animal production. As I discussed here, four times the amount of antibiotics was sold for use on chickens, pigs, cows and other food animals than for human medicine in 2011. The vast majority are used to promote more rapid animal growth or as a substitute for better management practices to prevent disease.
This type of routine, low-dose use of antibiotics creates a breeding ground for resistant bacteria, known as "superbugs". These superbugs can leave livestock operations via multiple pathways, contributing to the rise in antibiotic resistant infections:
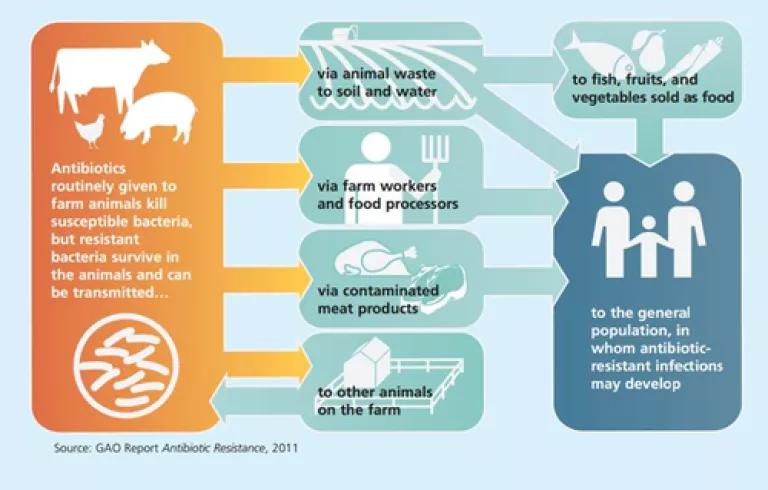
America's leading medical, scientific and public health organization agree that this overuse and misuse of important antibiotics in food animals must end in order to protect human health and have been ringing the alarm for years.
When institutions like these raise a red flag, it’s time to listen and act – not stick your head in the sand and hope the problem goes away.
Unfortunately, as my colleague Avi discussed here, FDA’s solution has been to offer loophole-laden recommendations to the drug and livestock industries and invite livestock producers to voluntarily reduce their use of antibiotics. FDA should be moving forward with mandatory action to stop the misuse of antibiotics in livestock production. The livestock industry keeps suggesting that there is not enough information to act. Scientists and the medical community say otherwise; there is more than enough information for us to act to address this health threat.
But more data is better; let’s gather it.
Improved monitoring and reporting requirements for antibiotic sales are critical to better understand the trends in antibiotic resistance and to track whether efforts to address this public health crisis are working to reduce antibiotics use and resistance. A broad coalition of medical, public health, scientific, agricultural, consumer, environmental, humane, and other organizations, representing more than 11 million supporters, has called for Congress and the FDA to create meaningful antibiotic use reporting requirements to address these basic data needs.
Despite the urgency of the situation, here are some of the things that we do not know:
Which drugs are being used on which animals. Currently, no reliable information is collected on the animal species in which the drugs are actually used except in the rare case where a drug is approved for a single species.
How the drugs are being used. FDA does not currently publicly disclose information on antibiotic sales by method of antibiotic administration (i.e., by feed, water, or injection) although it acknowledges that group delivery of antibiotics to large numbers of animals, such as via feed or water, poses a higher risk for breeding resistant superbugs.
For what purpose the drugs are being used. FDA does not disclose what information it has on purpose of use or on the amount of sales over-the-counter versus by prescription or other means.
How important the drugs being used are. FDA does not break out information on antibiotic sales by the medical importance of the drugs.
This week, Representatives Henry Waxman and Louise Slaughter took an important step in the right direction, by including new reporting requirements in the recently introduced and aptly named "Delivering Antimicrobial Transparency in Animals Act"—or “DATA Act.” The DATA Act would require drug companies to provide better information to the FDA on how their products are being used by the livestock industry and require FDA to provide more informative public summaries of both the new information and the data it already collects (but does not disclose). It would also require large poultry, swine and other livestock producers to report to FDA on the type and amount of antibiotics in feed given to their animals.
The DATA Act won’t solve the larger problem of antibiotic abuse in the livestock sector, but it is a common sense step towards better regulation of antibiotics in livestock to ensure that the effectiveness of these life-saving drugs is not being squandered by the livestock industry, putting the public health at risk. [Unfortunately, such common sense did not prevail in the Senate this week. Read about it here.]
The Obama administration should step up and support the DATA act. We need to ensure that our antibiotics work when we need them most and a lack of information on antibiotics use in the livestock industry can’t remain an excuse for inaction. Greater transparency in the administration of these precious drugs to food animals is critical to confronting this public health crisis.



