In late May, resistance to colistin—a last resort when other antibiotics fail—was first identified in bacteria from both a patient and pigs in the U.S. From its first discovery in China, the easily transmissible resistance to this drug spread rapidly around the world before reaching American soil.
Last month, I heard Dr. Beth Bell, a CDC expert in emerging infections, testify at the meeting of the President’s Advisory Council on Combating Antibiotic Resistant Bacteria that it was no longer a question of “if” but “when” we’d see more evidence that this deadly resistance had spread to more patients in the U.S.
The wait was disturbingly short. Yesterday, the journal Antimicrobial Agents and Chemotherapy published news of a second U.S. patient, this one in a New York hospital, infected with an E coli bug carrying this colistin resistance, conferred by a gene known as mcr-1 that sits on a plasmid—a string of DNA easily transferred amongst different bacteria. (In truth, we’d been expecting the news since Reuters gave a foreshadowing of it about two weeks ago.)
This is troubling because resistance genes to multiple antibiotics can collect on the same plasmid. Exposing bacteria to any one of those drugs hastens the likelihood they will acquire that plasmid and become capable of infections that resist treatment with any of the antibiotics. We are lucky there still is no sign of a plasmid that carries both colistin resistance, and resistance to other antibiotics of last resort. But when that happens (that’s when, not “if”), rising numbers of patient will die, infected by bacteria that are basically untreatable. In her recent story, Lena Sun of the Washington Post deems this future bacterium a scary “super-superbug”.
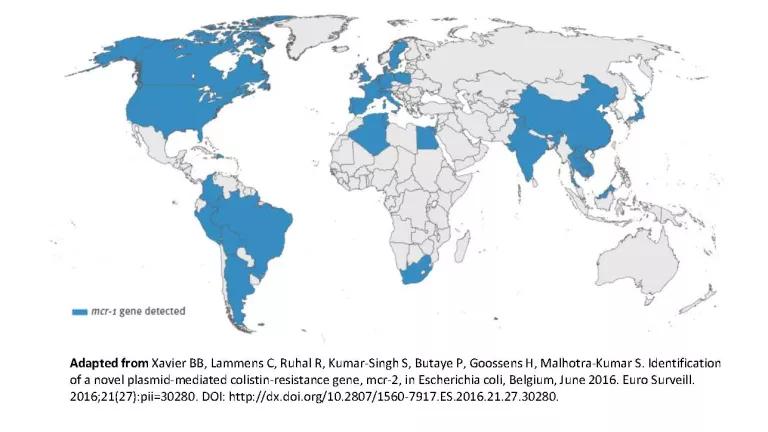
There’s no way to sugarcoat the bad news. The colistin resistance (mcr-1) gene, and our awareness of it, has traveled fast. Nine months after its initial discovery, scientists have identified the gene in human, animal and soil bacteria in 32 countries, as this adapted map shows.
In fact, the Iowa lab releasing yesterday’s study had tested more than 20,000 bacteria collected from hospitals around the world. Nearly 390 of these samples, or 2%, were resistant to colistin. The colistin-resistant E coli cultured from the New York patient was the only one of 15 such bacteria from the U.S. found associated that was with the mcr-1 resistance gene. (In contrast, all three cases of colistin-resistant bacteria from Madrid carried the gene, as did 5 of 10 German cases and 4 of 9 cases in Italy.)
The other shoe dropped with last week’s announcement of a second colistin resistance gene discovered in calves and pigs in Belgium. Like its close cousin, this gene (mcr-2) is carried on plasmids. However, the study suggest that mcr-2 may be several times more easily transmitted between bacteria than mcr-1. And it’s already more prevalent in Belgian pigs than is mcr-1. It's prevalence and transmissibility suggests mcr-2 may hasten the spread of colistin resistance, including via farms.
The discovery and spread of colistin resistance that has the potential to combine with other drug resistance and then spread easily between bacteria puts us in a brave new world. It’s not overuse of colistin alone that is the problem, however, but overuse of all antibiotics.
We know that antibiotic use and overuse provides the driver that spurs bacteria to evolve into superbugs. Conversely, if we could reduce our entire use of antibiotics, we would remove a lot of what drives resistance in the first place. Right now, 70 percent of the same antibiotics we rely on to treat sick people are sold for use on farm animals in the U.S. And the vast majority of those are distributed to animals en masse (i.e. via food or water) to speed up growth and as a poor substitute for better animal health and living conditions. That’s a foolish waste. In short, it is critical that antibiotic overuse in livestock be curtailed. In countries (Denmark and the Netherlands, for example) where use of antibiotics in livestock has already been reduced by 50 to 60 percent, public health gains have been significant.
So what are we waiting for? Every new delay in taking action to set targets, and achieve real reductions in antibiotic use in livestock only makes the future we all dread even more likely.



